Ionic Vs. Covalent Bond – What Are The Differences?
The ionic bond and covalent bond are different bonds in both structure and properties. The ionic bond is created when a positively charged ion and a negatively charged ion bond together.
The covalent bond has pairs of electrons that are shared by two atoms and they bind the atoms into a fixed orientation. It takes a certain level of electronegativity for two atoms to form a covalent bond.
What is an Ionic Bond?
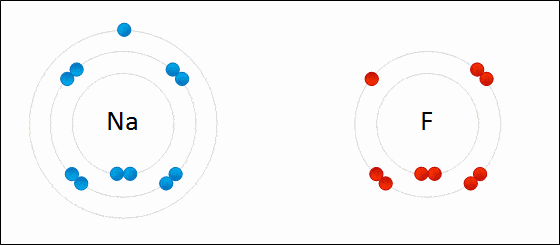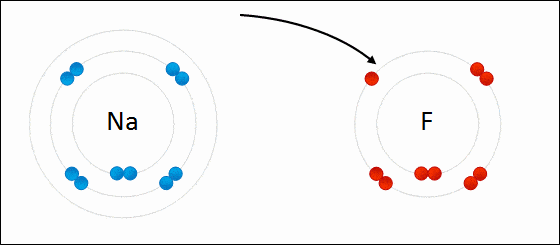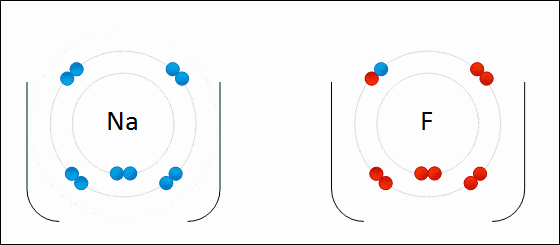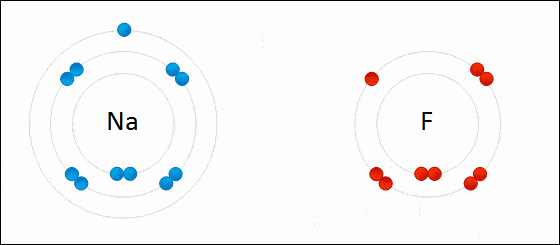
Ionic bond is also known as electrovalent bond and is a type of bond that forms from the electrostatic attraction between positive and negative charged ions.
Mainly form between metal compounds and non-metal compounds. The melting point is high. The boiling point is also high.
How is Ionic Bonds Formed?
It is formed when a metal and non-metal combine. Non-metals are considered to be stronger than metals. The non-metals can easily take electrons from metals.
The two opposite ions then attract to each other and form an ionic bond. Polarity is high. Ionic bonds will have no definite shape. Ionic bonds are solid at room temperature.
What is a Covalent Bond?
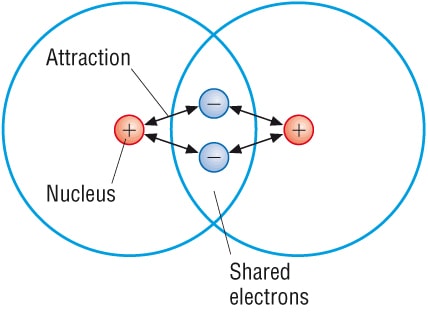
A covalent bond is a form of chemical bonding between two atoms of non-metals. It is characterized by the sharing of electrons between other covalent bonds. The melting point is low. The boiling point is also low.
How are Covalent Bonds Formed?
Two non-metals that have similar electronegativities will attract each other. Neither atom has the strength to take an electron from the other.
Therefore, to become stable they will share electrons from the outer molecular orbit with each other.
This forms a covalent bond. Polarity is low. Covalent bonds do have a definite shape. Covalent bonds are liquid or gas at room temperature.
Recommended for You:
Ionic Bonds Vs. Covalent Bonds
Here are 4 differences between the ionic bonds and covalent bonds.
Ionic Bonds |
Covalent Bonds |
|
|
|
|
|
|
|
|